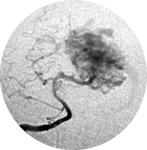
 Brain arteriovenous malformation
Brain arteriovenous malformation
V. Pathophysiological principles of the pathological process
*1. Etiology
The true pathogenesis (when lesion formation is initiated, how it typically progresses, or how progression is impacted by environmental risk factors) of AVMs is unknown and the currently available animal models of cerebrovascular malformation still have limitations. [13, 48]
Many authors believe that cerebrovascular malformation arises during embryonic development and two main hypotheses for AVM pathogenesis include: embryonic agenesis of the capillary system and the retention of a primordial connection between arteries and veins. [30] However these hypotheses are not proved, and little direct evidence supports these ideas.
As proposed by Leblanc et al. [48] some (perhaps most) cerebrovascular malformations arise post-natally and into adulthood. They believe that studying cerebrovascular malformation genes function in adult angiogenesis and vascular repair and how their expression is affected by environmental perturbations will help in the understanding of AVM etiology. As said in epidemiology, AVMs typically present at a mean age of 33 years, and not in childhood as should be expected in congenital lesions [20, 103]. In addition, there is clear evidence that AVMs are dynamic and biologically active lesions rather than static congenital vascular abnormalities, with growth or regress, changes in their structure and form again later in life [17, 30, 65].
AVMs can occur either sporadically or in the context of genetic syndromes. The primary one associated with cerebral AVM is hereditary hemorrhagic telangiectasia (HHT) [48]. Nevertheless, AVMs may be associated to other well-defined genetic diseases: Osler-Weber-Rendu disease, telangiectasia ataxia, Wyburn-Mason syndrome, and Sturge-Weber syndrome [42, 44, 67].
More than 900 genes are known to be involved in the pathogenesis of AVMs and their molecular characterization, pattern definition, and family heritage relationships are a challenge [67]. Some genes highlighted by Leblanc et al. [48] are Endoglin (ENG), Activin-like kinase receptor 1 (ACVRL1; ALK-1) and SMAD4. They propose that HHT Types 1 and 2 result from loss-of-function mutations in one copy of the Endoglin (ENG) and Activin-like kinase receptor 1 (ACVRL1; ALK-1) genes, respectively. ALK-1 variants may also be associated with risk for sporadic AVM [82]. The SMAD4, were described in some cases of combined juvenile polyposis and HHT syndrome [22].
About the formation of AVMs there is a more current hypothesis of the genetic “two-hit” mechanism, in which an inherited mutation in one copy of a cerebrovascular malformation gene is followed by a somatic mutation in a second copy [48, 55]. As observed by Leblanc et al. [48], alternatively, the second “hit” could be environmental in the form of a localized physiological or pathological perturbation. There is an interaction between genetic factors related to vasculogenesis and hemodynamic factors. The development of AVMs is related to genetics, a number of growth factors and extracellular proteins that may inhibit or stimulate their growth and reshaping, besides their structural and hemodynamic features.
 Encyclopædia Neurochirurgica
Encyclopædia Neurochirurgica

