 Diffuse low-grade gliomas
Diffuse low-grade gliomas
SUMMARY
Diffuse low-grade gliomas (WHO grade II) are a sub-group of rare and heterogeneous primary brain tumors that usually occur in young patients living a normal life until the onset of a first seizure.
A good understanding of the natural history of these gliomas namely: their steady progression, infiltration along white matter fibers and especially the risk of malignant transformation-which endangers the functional and vital prognosis, associated with the minimization of the risk of treatment, has led to a therapeutic change from the “classic” conservative attitude to a more rigorous therapeutic strategy.
Current goal is to elaborate dynamic and individualized treatment; that is; to define the sequence and timing of each treatment option (single to multiple safe maximal surgical resections within cortical-to-sub-cortical functional borders, single to multiple chemotherapy and radiotherapy sessions) depending on tumor progression (measured on regular follow up MRI), clinical and neurocognitive status and individual’s functional anatomy of the brain (studied via brain mapping and susceptible to reorganization through the phenomena of neuroplasticity) to prevent malignant transformation as long as possible while preserving the quality of life.
Only a multidisciplinary approach to multi-center networks can afford to give a real future to patients with this chronic brain disease, with the possibility to design long-term projects be them socio-professional or at the household level. The next step would be that of early screening in order to provide preventive treatment.
Key Words (MeSH): Low-grade gliomas, Surgery, Chemotherapy, Radiotherapy, Quality of life, Molecular biology
I – DEFINITION
Low-grade gliomas are primary central nervous system tumors arising from glial cells and comprise of World Health Organization (WHO) grade I and II gliomas (44).
The former include pilocytic astrocytomas, gangliogliomas, subependymal giant cell astrocytomas, dysembryoplastic neuroepithelial tumors, and most recent group considered as mixed neuroglial tumors (44).
There are usually benign pediatric tumors and well -delineated within the brain parenchyma. Surgical excision alone may lead to complete remission for decades and even a real cure.
Conversely, grade II gliomas, namely adult supra-tentorial diffuse low-grade gliomas (DLGG), due to their constant growth, infiltrating nature and inevitable malignant transformation are more difficult to understand.
In essence, DLGGs, which include astrocytomas, oligodendrogliomas and grade II oligoastrocytoma, are heterogeneous entities involving complex brain tumors with different clinical, radiological, histological and molecular characteristics. They should no longer be considered ’benign’ tumors, but as ’pre-malignant’ cancerous tumors.
Thanks to the understanding of their natural history the therapeutic strategies have changed radically in recent years, currently leading to individualized strategies based on early and repeated therapies - rather than a simple follow up as long advocated (26). Thus, this chapter will be purposely devoted on adult DLGG.
II – EPIDEMIOLOGY
DLGGs represent approximately 15% of gliomas, with an incidence of approximately 1/100 000 per year, and a prevalence of 9/100 000 (60). The median age of patients is between 35 and 40 years. Indeed, in a recent series of the French Network for the Study of gliomas (Réseau Français d’Etude des Gliomes =REG) on 1091 patients, the median age at diagnosis was 37 years (8).
The sex ratio (male / female) is estimated around 1.32 (60), with a predominance of DLGG in Caucasian men. Exposure to ionizing radiation is the only known environmental risk (64). None of the other risk factors considered in theory (chemicals, mobile phones, hereditary factors, etc ...) have never been demonstrated as critical in the development of a DLGG - whose origin remains unknown. Worth noting is the probable inverse relationship between allergies and risk of developing a glioma.
III – NATURAL HISTORY
DLGGs most often affect young patients leading a normal family, social and professional life. Seizures occur most frequently at presentation of these tumors (71). They are often located within or close to ’eloquent’ brain areas involved in sensorimotor, language, visuospatial, memory or cognitive functions.
Despite the intrinsic variability of the biological behavior of these tumors, the natural history is now better known. It has long been claimed that the DLGG tumors were ’benign’, with little or no progression.
Recent studies have revealed the constant evolution of DLGG by demonstrating that these tumors:
(i) have a continuous spontaneous growth;
(ii) infiltrate brain parenchyma along the white matter tracts, and beyond the borders of abnormal MRI signal;
(iii) will unavoidably transform into high-grade glioma (46,56) (Figure 1).
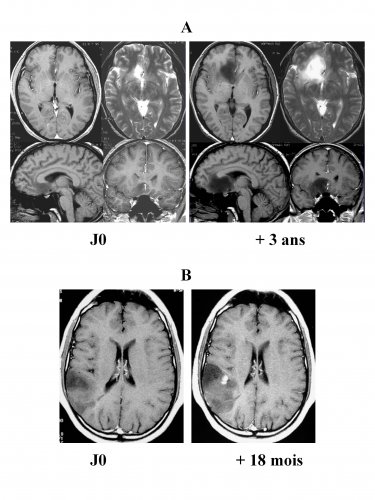
Natural history of DLGGs
A: Successive MRI showing an unavoidable growth – volume is 4 times higher three years later in an asymptomatic patient that is followed up.
B: Malignant transformation with the appearance of contrast enhancement 18 months after the onset of the first seizure in a patient that hasn’t received an oncologic treatment.
Thus, the concept of ’benignity of DLGG’ has been abandoned.
Two distinct periods make up the natural history of DLGG: a premalignant first phase starting with an occult period followed by a period of clinical and radiological visibility;
a second phase during which a grade II glioma undergoes genetic modification resulting in malignant transformation into a WHO grade III or IV glioma.
Beyond this dichotomous view based on a well distinct histological classification, there is actually a continuum between these evolutionary phases, during which the acquisition of genotypic and phenotypic characteristics of malignancy is progressive. The difficulty thus is to determine, for a given patient at a given moment, at what level (s)he is in the grade II - Grade III / IV transition (61).
In the premalignant period, linear growth of about 4 mm in average diameter per year (calculated on two successive MRI separated by at least three months interval, according to a methodology detailed in the chapter on imaging), was found in all cases, both in symptomatic patients as well as in incidentally discovered DLGG (53).
In fact, the concept of ’progression free survival’ has no meaning in untreated or incomplete surgically resected DLGG since by definition all DLGG continuously growing (although the concept remains valid after of total resection on MRI or in case of stabilization by chemotherapy and / or radiotherapy adjuvant treatments). In this context, conventional radiological criteria originally proposed by McDonald and more recently by the RANO group (75) are not suitable for DLGG, since they are based on the calculation of two diameters and not on the volume (with subsequent comutation of the mean diameter).
If the ’date of birth’ (tumour onset) of DLGG is not well known, recent biomathematics models have suggested the existence of two types of gliomas: the first corresponds to very slowly growing tumours that appear during adolescence, and the second type corresponds to slowly growing tumours that appear later, during early adulthood (32).
The long standing asymptomatic nature of DLGG as well as the rarity or extreme discretion of neurological dysfunction is explained by two mechanisms: (i) infiltration of the cerebral parenchyma by isolated tumor cells allowing the persistence of functional tissue within the tumor; (ii) the slow tumor progression enabling the establishment of a cerebral adaptation phenomena of neuroplasticity resulting in a dynamic reorganization of functional networks invaded by glioma (19).
Moreover, not only are these lesions progressively enlarging but they also migrate along the white fibers. Therefore, a DLGG is not a ’tumoral mass’ but a chronic disease progressively infiltrating brain parenchyma, particularly subcortical connections. It is this diffusion that eventually induces neurocognitive disorders, because (at least partly) of a very probable disconnection syndrome (27).
Finally, DLGG inexorably evolve towards malignancy. This transformation can be observed clinically and radiologically in a slow or rapid manner, again stressing the biological diversity of DLGG and explaining the possibility of diagnosing them at intermediate stages (ie not fitting perfectly in grades II or III / IV of WHO classification, but for example corresponding to a grade II with foci of anaplasia). The exact mechanisms involved in malignant transformation are currently unknown, preventing predictions of its occurrence at the individual level, even if genetic changes are readily associated (40).
It is worth noting the exceptional degeneration of small volume DLGG (less than 10 cc) (4). Clinically, the functional consequences of such a transformation are significant with onset and / or worsening of seizures, occurrence of neurologic deficits or intracranial hypertension. In addition, is associated radiological acceleration of tumor growth as well as appearance of a contrast enhancement, edema or mass effect and possible necrosis. The affection of the functional outcome results from the involvement of a functional area by three mechanisms which may occur in combination: (i) speed of tumor growth greater than the capacity of brain plasticity; (ii) an increase in the mass effect on the eloquent areas adjacent to the glioma and previously recruited by the phenomenon of neuroplasticity; (iii) destruction of axonal networks infiltrated by the tumor.
In all cases this transformation results in the patient’s death, with an overall median survival of less than 8 years for DLGG. Indeed, in a prospective randomized EORTC study involving over 600 patients, in the subgroup of patients with a favorable prognostic score, the median survival was 7.7 years (while it was only 3.2 years in the subgroup of patients with unfavorable prognostic score) (57). More recently, a comparative study between surgical excision and biopsy showed that the median survival was only 5.8 years in the group of patients biopsied (38). These data clearly demonstrate that the spontaneous prognosis of DLGG is relatively poor.
 Encyclopædia Neurochirurgica
Encyclopædia Neurochirurgica

