 Pediatric Medulloblastomas
Pediatric Medulloblastomas
VII. Treatment
The standard treatment is tumor resection, irradiation of the entire neuraxis except in children under 5 years old, and chemotherapy according to different modalities. So far, stratification into risk groups has been based on age and the presence of residual tumor and / or metastasis. The distinction of five histologic subtypes and the contribution of molecular biology has enabled the current definition of new paradigms for the multidisciplinary management of this disease (20). Finally, a better understanding of the molecular mechanisms underlying the development of the different types of medulloblastomas offers the prospect of targeted molecular therapies being tested in some patients with tumor relapse.
The classification used in most published series is that of Chang (12) based on a review of operative-records and autopsy performed since 45 years. This classification ought not to be used because of advances in imaging, the lack of impact of tumor size on survival (T) or differentiation between brain or spinal metastasis (M2 or M3). New classifications have been proposed (18), but none is currently the reference. The most important issue is to clearly precise on the quality of surgical excision by analyzing the operative report and MRI performed within 48 hours after excision.
Medulloblastomas are currently distinguished into 2 groups:
- ’Standard Risk’ Medulloblastomas
Total or subtotal resection (no residual tumor identified on post-operative MRI, or tumor residue with surface area of <1.5 cm² on MRI axial cuts) or absence of supratentorial or spinal metastases; no meningeal spread ;
![]() ’High Risk’ Medulloblastomas
’High Risk’ Medulloblastomas
Residual tumor> 1.5 cm² , and / or existence of supratentorial or spinal metastases and / or the presence of an invasion of the cerebrospinal fluid.
Molecular biology classification (Table I)
Table I. Classification of medulloblastomas using molecular biology (4 groups)
Wnt (15%) |
Shh (25%) |
Groupe 3 |
Groupe 4 | |
| Histology | Classic, rarely large cell / anaplastic | Classic, large cell / anaplastic, desmoplastic / nodular | Classic, large cell / anaplastic | Classic, large cell / anaplastic |
| Metastases | Rares | Less frequent | More Frequent | Frequent |
| Prognosis | Very good | Good in infants, intermediate in the aged | Poor | Intermediate |
| Genetics | Mutation of CTNNB1 | Mutations of PTCH1/ Smo/ SUFU; amplification of Gli2; amplification of NMYC | amplification of MYC | Amplification of NMYC and CDK6 |
Therapeutic management is also guided by the age of the child. For younger children, current studies seek to avoid or delay the CNS irradiation because of the deleterious effects of radiation on the developing nervous system (see below). The age limit below which the irradiation of the entire CNS is contraindicated depends on the results of ongoing studies; and is currently set at five years.
Preoperative workup includes ophthalmic examination to detect signs of intracranial hypertension (especially papilledema) and serves as a baseline for follow up.
1. Surgical Treatment
- Principles
Treatment of hydrocephalus
Tumor resection can not be performed in the presence of significant intracranial pressure observed in 60-80% of cases. Treatment of hydrocephalus must always be discussed. Treatment by endoscopic third ventriculostomy (ETV) is gradually replacing the external drain and ventriculo-peritoneal shunt. A CSF sampling should be routinely done for cytology if treatment of hydrocephalus is necessary
Surgical Treatment
Surgery involves tumor resection from the posterior fossa and, if necessary, to treat hydrocephalus before this procedure. However, if there are clinical signs and / or significant radiological signs of herniation at the foramen magnum (Figure 7), surgical resection should be performed urgently, with an external ventricular drain inserted at the end of surgery (to avoid death from acute postoperative hydrocephalus).
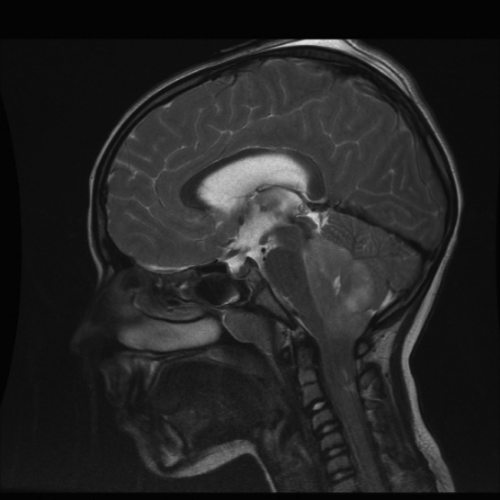
Figure 7. Surgical emergency. Brain MRI: T2-weighted image Mid-Sagittal cut,. Voluminous posterior fossa lesion causing foramen magnum herniation and cranio-cervical junction compression.
The posterior fossa (PCF) approach used is not specific for MB surgery. Details wouldn’t be discussed here, but apply to any midline PCF tumor. It is done in lateral decubitus or sitting position; the latter preferred by the authors. (Figures 9 and 10). Indeed, the authors observed fewer complications as in other series(50). However it is worth precising certain principles that must be kept in mind during this surgery:
![]() Special attention must be paid to the cerebellar vermis, as it plays an important role in the cognitive processes (41, 62) and vermian lesions are widely incriminated in subsequent neurocognitive sequelae (55). It is therefore appropriate to prefer the telo-velar approach (15, 46) to a vermian incision. This approach allows for a better exposure angle in all planes compared to the transvermian (except for the rostral part of the fourth ventricle, but the removal of the posterior arch of C1 optimizes the quality of exposure). In addition, in order to limit the deleterious compression on the dentate nuclei, we recommend using only one retractor, which in principle should be inclined on one or the other cerebellar tonsil to access the tumor.
Special attention must be paid to the cerebellar vermis, as it plays an important role in the cognitive processes (41, 62) and vermian lesions are widely incriminated in subsequent neurocognitive sequelae (55). It is therefore appropriate to prefer the telo-velar approach (15, 46) to a vermian incision. This approach allows for a better exposure angle in all planes compared to the transvermian (except for the rostral part of the fourth ventricle, but the removal of the posterior arch of C1 optimizes the quality of exposure). In addition, in order to limit the deleterious compression on the dentate nuclei, we recommend using only one retractor, which in principle should be inclined on one or the other cerebellar tonsil to access the tumor.
Figure 9. Sitting position for posterior fossa surgery. Marking of incision Line (from the external occipital protuberance to the spinous process of C2).
Figure 10. Sitting position for posterior fossa surgery
The telo-velar approach requires the removal of the posterior arch of C1, which wouldn’t affect the stability of the cervical spine, as long as the integrity of C1-C2 ligaments are respected
![]() The trans-vermian approach are to be avoided, if not proscribed.
The trans-vermian approach are to be avoided, if not proscribed.
- Results
Tumor excision should:
![]() Allow for the diagnosis of medulloblastoma
Allow for the diagnosis of medulloblastoma
![]() Be as complete as possible (a residue <1.5 cm² however is of little impact on prognosis)
Be as complete as possible (a residue <1.5 cm² however is of little impact on prognosis)
Consequences (side effects, complications)
Complications of the PCF surgery are:
![]() Mutism
Mutism
![]() Posterior fossa Syndrome (its incidence is similar to that of cerebellar mutism and be prevented intraoperatively: see previous paragraph).
Posterior fossa Syndrome (its incidence is similar to that of cerebellar mutism and be prevented intraoperatively: see previous paragraph).
![]() CSF Leak
CSF Leak
![]() Infections (local, abscesses, meningitis ...)
Infections (local, abscesses, meningitis ...)
![]() Wound gapping
Wound gapping
![]() Acute hydrocephalus
Acute hydrocephalus
![]() Neurocognitive alterations (for affection of the fronto-cerebellar pathways) (65).
Neurocognitive alterations (for affection of the fronto-cerebellar pathways) (65).
POST OERATIVE WORKUP
It should be done in the immediate postoperative period, within 48-72 hours after surgery. MRI done after this period is of no value because it does not distinguish a residual tumor from post-operative bleeding or even a post-operative scar. It is aimed at detecting the residual tumor in the posterior fossa and should include MRI with and without contrast injection.
CSF examination from a lumbar puncture between the seventh and the fifteenth day after surgery aims at detecting subarachnoid dissemination by cytocentrifugation of CSF and measurement of the protein level.
MRI or CSF cytology alone does not completely rule out metastatic spread but these two investigations should be systematically done.
.
BIOLOGY AND MOLECULAR BIOLOGY
After tumor resection, some laboratory tests should be routinely done to stratify the disease and define the therapeutic strategy. Immunohistochemical examination may reveal β-caténine which is of good prognostic value. (19).
Preservation of tumor samples in a tumor bank after freezing to - 80 ° C is essential to study the prognostic value of various biological parameters.
Molecular biological studies should investigate for the amplifications of C-Myc and N-Myc oncogenes that are of unfavorable prognosis and most often seen in the anaplastic / large cell types.
Furthermore, a detailed analysis by different methods of molecular biology has clarified the embryonic tumorigenesis of medulloblastomas to enable molecular classifications that have been developed over the years. Thus, the normal development of the cerebellum requires the activation of signaling pathways including sonic hedgehog pathway (Shh) and wingless (Wnt) (58) pathway. A mutation in these pathways results in the development of the Shh subtype of medulloblastoma, , mainly located at the 4th ventricle and Wnt subtype type generally located in the cerebellar hemispheres. The current classification recognizes four risk subgroups (Wnt, Shh, Group 3 and Group 4) takes into consideration the demographic, clinical presentation, histology and genomic profile (47). These subgroups Shh, Wnt and non-Shh / Wnt respectively account for approximately 25%, 15% and 60% of medulloblastomas (61).
The Shh subtype is mainly in children less than 3 years; its histology essentially corresponds to the nodular / desmoplastic form and the prognosis is good in infants, intermediate in older children.
In the Wnt subtype; classical histological form is common; it is occurs at an older age; it rarely metastasize and prognosis is very good.
In group 3, the tumors are often metastatic, the histology is of the anaplastic or large cell type, and occurs in younger children with a masculine preponderance. The prognosis of this group is the most pejorative.
Group 4 involves mainly younger children; metastatic forms exist but are less frequently than in group 3, histology is essentially that of the classic type. The prognosis is intermediate.
The histologic differential diagnosis of medulloblastoma includes other posterior fossa tumors such as the ependymoma with a different histological appearance (perivascular arrangement of rosettes providing a fibrillar space network around the vessels) and especially the ependymoblastoma, another PNET arising from fourth ventricle and having an ependymal differentiation. The therapeutic management of these two primitive neuroectodermal tumors of the posterior fossa is currently the same.
2. Radiotherapy
Medulloblastoma is a highly radiosensitive tumor. The high propensity of medulloblastoma to spread across the CNS and the failures of radiation therapy focused to the posterior fossa have led to radiotherapy of the entire central nervous system. The latter is done using rigorous techniques in order to limit the risk of overdose to the cord and underdosing of the subarachnoid space. Conventionally 54 Gy is delivered in 30 fractions of 1.8 Gy to the tumor bed; from 25-36 Gy in 12-18 fractions of 1.8 Gy on the brain and the spinal axis.
Brain metastases can receive focal irradiation of 45-54 Gy (depending on the target volume); spinal metastases above L2 focal irradiation of 39.6 to 45 Gy (also depending on the target volume) and those below L2 focal irradiation of 50.4 to 54 Gy.
This should be done with extreme rigor and quality control including a review of centering layouts necessary for precise irradiation (10). New technologies such as tomotherapy facilitate the technique. Similarly, proton therapy, although still with limited access in France, are advantageous in the protection of the auditory apparatus. RT is done as soon as possible after surgery or after chemotherapy which can be intercalated (’sandwich’ chemotherapy). In this case, the time between surgical excision and radiotherapy, defined in each protocol is in the order of about 90 days. The surgery-radiotherapy time interval and the duration of radiation therapy are essential prognostic factors. It has been shown in the SIOP II study comparing the cranio-spinal irradiation (CS) in which irradiation with 36 or 23,4Gy, is or is not preceded by chemotherapy that the results were worse for the group of patients who underwent a pre-radiotherapy chemotherapy due to a delay in radiotherapy (2).
If the hematologic profile has been altered by chemotherapy, then radiotherapy can start in the posterior fossa if there is no metastasis or meningeal dissemination to avoid hematotoxicity due to irradiation of the spinal axis .
In rare occasions, radiation therapy may cause a surge in intracranial hypertension, and / or exacerbate pre-existing neurological signs. This complication usually responds well to corticosteroids.
Sequelae caused by the RT led to a change in the modalities for irradiation in the standard risk group. Reducing the cranio-spinal dose to 23.4 Gy without chemotherapy has been the subject of several trials with conflicting results: no loss of opportunity in the SIOP II study (2), and excess spinal recurrences in the POG8631 / CCG923 study yet without significant difference in survival at eight years (67). However, in most studies, the association of low dose cranio-spinal irradiation to chemotherapy improves the prognosis with a five-year disease-free survival of 75 to 80% (51, 68).
A bi-fractionated radiotherapy was also tested in order to reduce the irradiation sequallae in the MSFOP 98 study that delivered a dose of 36 Gy to the cranio-spinal axis without associated chemotherapy. Overall survival at six years was 78% and progression-free survival of 75% (11). The results in terms of cognitive function are promising. In the randomized study of the International Society of Pediatric Oncology (SIOP) comparing hyperfractionated versus conventional radiotherapy followed by chemotherapy, there was no difference in event-free and overall survival nor in terms of auditory toxicity (40).
Toxicity of the auditory apparatus that occurs in about two thirds of cases appears between 50 and 60 Gy. Reducing the volume irradiated in the posterior fossa limits the toxicity. This was shown in a study comparing conventional radiation and intensity modulated RT (IMRT) in which 84% of patients treated conventionally had an auditory toxicity (64% grade 3-4) against 27% in the IMRT group (including 13% grade 3-4) (34). It has recently been shown that the use of proton therapy is also possible to reduce the frequency and severity of radiation-induced ototoxicity (43).
3. Chemotherapy
Medulloblastoma is the most chemosensitive CNS tumor after germ cell tumors as shown by the response rate observed in the residual tumor or recurrent forms (13). These rates vary between 60 and 90% according on the results of Phase II studies where the number of evaluable patients, was however, between ten and thirty. The best response rates were obtained with polychemotherapy with a platinum derivative. Combined chemo- and radiotherapy has increased survival rates and reduced the doses of prophylactic radiotherapy from 35-25 Gy in standard risk patients (51). In children less than 5 years it has also permitted to defer radiotherapy or avoid it in some low-risk patients (27, 59).
Successive clinical trials and advances in biology have enabled a distinction in treatment groups according to age of the child, the grade, the quality of excision and biology of medulloblastoma.
For children over 5 years of standard-risk group (localized, no tumor residue, not of unfavorable biology), the use of 8 cycles of poly chemotherapy with cisplatin CCNU and vincristine is currently the standard treatment after radiotherapy. This treatment gives a 5 years survival rate of about 85% (40).
There is no defined standard treatment for high-risk patients. Besides surgery and cranio-spinal irradiation, other treatment modalities include, conventional or increasingly high dose chemotherapy followed by autologous hematopoietic stem cells (HSC) transplantation. Encouraging results have been reported with a recurrence-free survival rate of 70% at 5 years (22).
With regard to children under 3 to 5 years of standard risk, several chemotherapy protocols have been tested to defer or even avoid irradiation. Most of these protocols use some or all of the following molecules: vincristine, cisplatin, carboplatin, etoposide, cyclophosphamide, and methotrexate.
In BBSFOP study, 22% of children were alive at 5 years with prolonged postoperative chemotherapy without radiotherapy. Five year progression-free survival was 41% in the R0M0 group with complete resection versus 0% in case of subtotal resection (27). In this group, most relapses occurred in the posterior fossa and were treated with conventional chemotherapy and high-dose chemotherapy followed by transplantation of HSCs and eventually by resection and radiotherapy to posterior fossa only in case of local recurrence, to obtain an overall 5-year survival of 73%.
In the German HIT SKK study combining conventional chemotherapy, administration intraventricular methotrexate and systemic high dose methotrexate , event-free survival at 5 years was 83% (59). These studies clearly identify prognostic factors for medulloblastomas in children under 5 years, with worst prognostic factors being: histologic classic or anaplastic / large cells types, the existence of a residual tumor and metastases whereas the best survival rates were observed among the cases of medulloblastoma of desmoplastic / nodular or extensive nodularity types, localized tumors, without residue or metastasis (60). It has been shown, via remotely conducted neuropsychological tests that intellectual sequelae were significantly lower among children who had not received craniospinal irradiation.
In case of relapse or progression, it is possible to propose the inclusion in Phase II trials of metronomic chemotherapy with methotrexate-vinblastine-celecoxib-cyclophosphamide (CECS-Metro 01) or topotecan + temozolomide (TOTEM 2).
 Encyclopædia Neurochirurgica
Encyclopædia Neurochirurgica